 The Role of Sterilization Labels in the Healthcare and Pharmaceuticals Industry
The Role of Sterilization Labels in the Healthcare and Pharmaceuticals Industry
In the healthcare and pharmaceutical industries, safety, precision, and compliance are not just operational goals—they're non-negotiable standards. From surgical tools to pharmaceutical packaging, every component in the chain must pass rigorous sterilization processes to ensure patient safety and product efficacy. But how do hospitals, labs, and pharmaceutical facilities track and verify sterilization?
This is where sterilization labels play a silent yet critical role.
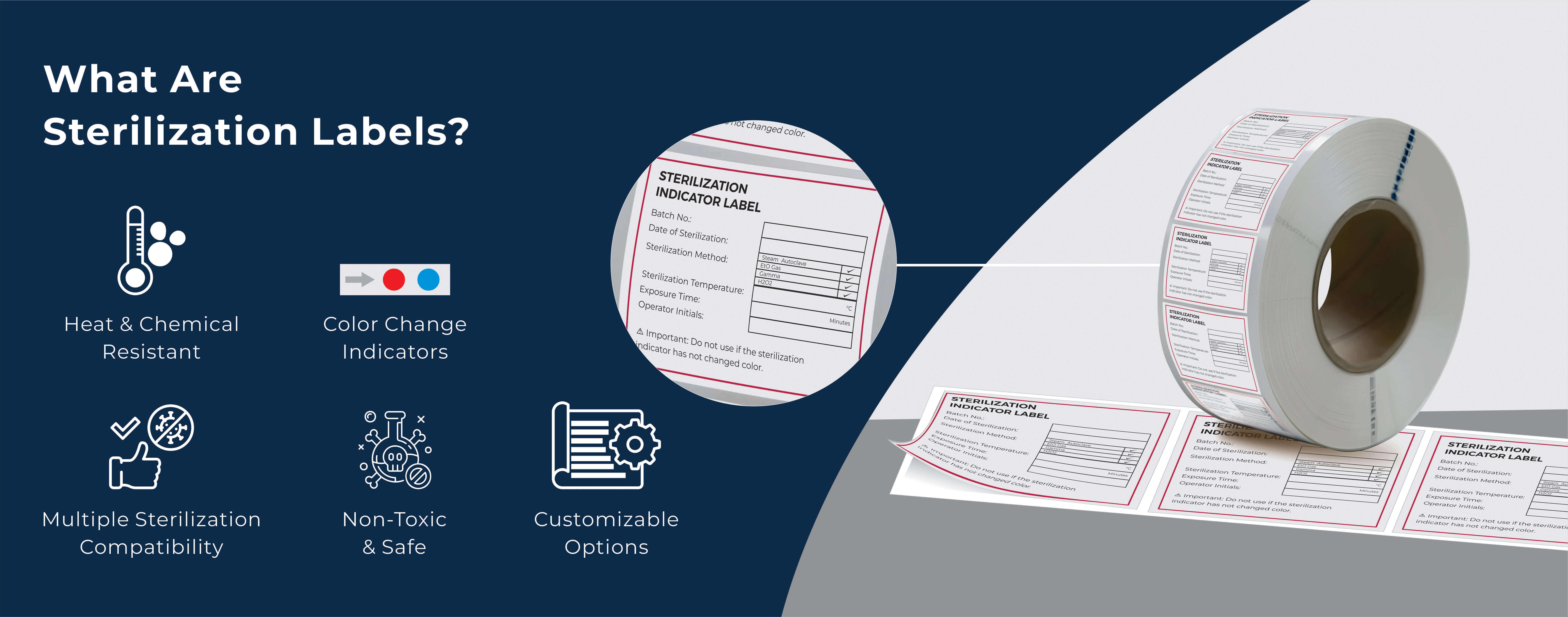 What Are Sterilization Labels?
What Are Sterilization Labels?
Sterilization labels are specialized adhesive labels designed to withstand high temperatures, pressure, and chemical exposure during sterilization processes. Beyond their durability, they also function as visual indicators that confirm whether an item has undergone sterilization.
Whether it’s steam autoclaving, ethylene oxide (EtO) sterilization, or gamma irradiation, these labels change color or display specific signals once a sterilization cycle is complete—helping frontline medical workers quickly identify whether a pack, tray, or tool is safe for use.
 Why Sterilization Labels Matter
Why Sterilization Labels Matter
In fast-paced hospital environments or high-volume pharma production lines, clear visual communication is essential. Sterilization labels reduce guesswork and human error, ensure compliance with health regulations, and prevent the use of non-sterile items.
Let’s look at why they’re indispensable in these sectors.
1. Infection Prevention and Patient Safety
A single lapse in sterilization can result in life-threatening infections. Sterilization labels act as the first visual checkpoint that tells medical staff if an instrument is safe to use. This minimizes the risk of cross-contamination, especially during surgeries or in sterile environments like ICUs.
These labels are particularly crucial in:
-
Operating Theatres – to mark sterilized instrument trays.
-
Dentistry – for labeling hand tools, surgical packs.
-
Diagnostic Labs – to distinguish between sterile and used pipettes, petri dishes, or containers.
2. Compliance with Regulatory Standards
Hospitals and pharmaceutical manufacturers are bound by global sterilization standards like ISO 11140, FDA guidelines, and WHO protocols. Sterilization labels provide a traceable, verifiable method to document sterilization—making audits and quality control checks smoother.
For example:
-
Color-changing indicators on labels show whether the item has passed through the required temperature or chemical conditions.
-
Labels with batch numbers and timestamps help track cycles in digital records.
-
They reduce the risk of non-compliance during FDA, NABH, or ISO audits.
3. Process Validation in Pharmaceuticals
In the pharmaceutical industry, packaging materials, tools, and even raw materials often undergo sterilization before entering cleanrooms. Sterilization labels help validate that every stage has been completed as per SOPs.
Applications include:
-
Labeling of autoclaved packaging used for vials, syringes, or ampoules.
-
Tracking sterilized mixing tools or containers in production.
-
Documentation for batch release records, essential for GMP compliance.
 4. Resistance to Harsh Environments
4. Resistance to Harsh Environments
Sterilization isn’t gentle. Steam autoclaves can reach temperatures up to 134°C, EtO involves toxic gases, and gamma sterilization uses radiation. Labels used here must survive without smudging, detaching, or losing legibility.
High-performance sterilization labels are:
-
Waterproof and chemical resistant
-
Made with strong adhesives that don't degrade
-
Printed with inks that withstand extreme heat and pressure
They continue to adhere securely and remain legible after the process—ensuring reliable tracking from sterilization to point-of-use.
5. Integration with Hospital Workflow & Automation
Modern healthcare systems use barcode scanning and RFID tagging to improve efficiency. Sterilization labels now come with pre-printed barcodes, or blank surfaces compatible with thermal printers, allowing:
-
Scan-and-track features in hospital inventory systems
-
Real-time updates to sterilization logs
-
Automation-friendly formats for print-and-apply label systems in pharma facilities
6. Customization for Application-Specific Needs
Not all sterilization labels are one-size-fits-all. A syringe label for pharmaceutical use differs vastly from a surgical tray label. Leading converters like Monarch Graphics provide:
-
Labels designed for EtO, steam, or gamma sterilization
-
Customized sizes, shapes, and adhesives for specific instruments or packs
-
Multilingual labeling, traceability codes, and regulatory content pre-printed for pharma packaging
This level of customization ensures that the label adds real operational value.
 Monarch Graphics: Enabling Safer Sterile Practices
Monarch Graphics: Enabling Safer Sterile Practices
At Monarch Graphics, we understand the critical role labeling plays in maintaining sterile supply chains. Our sterilization labels are:
-
Tested for use in extreme environments
-
Compatible with industry-leading sterilizers
-
Made from medical-grade materials
-
Fully compliant with healthcare and pharma labeling norms
Whether you're a hospital looking for reliable surgical labels or a pharma manufacturer needing validated labels for sterile production, our team can help you choose or develop the right solution.
Conclusion
Sterilization labels may seem like small components, but they have big responsibilities—ensuring safety, compliance, and operational efficiency in two of the most critical industries: healthcare and pharmaceuticals.
They act as silent sentinels—flagging whether a tool is ready to save lives, or if a package has passed all required sterilization stages before it reaches the patient.
Need help upgrading your sterilization labeling process? Contact Monarch Graphics today for custom, regulation-ready sterilization label solutions.


